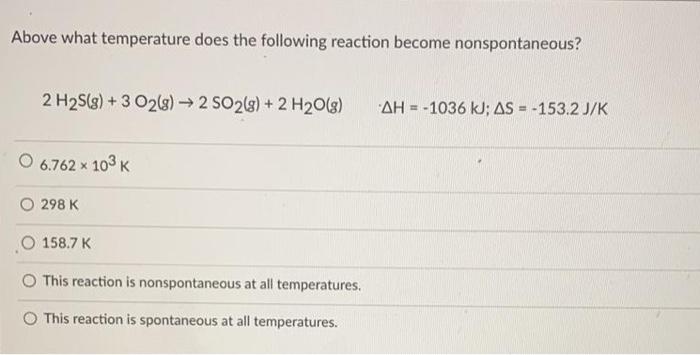
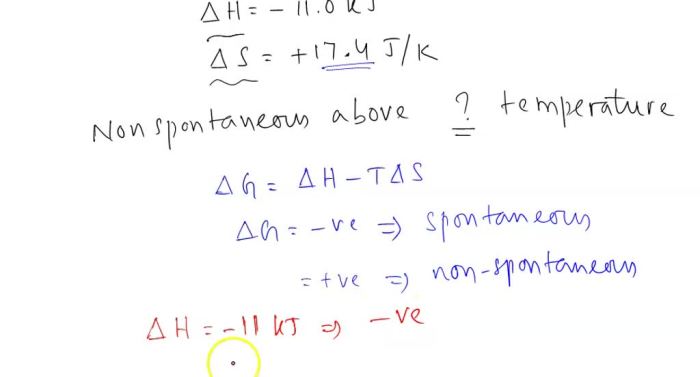Above what temperature does the following reaction become nonspontaneous – Exploring the concept of nonspontaneous reactions, this article delves into the fascinating relationship between temperature and reaction spontaneity. We will examine the factors that influence a reaction’s spontaneity and explore methods for determining the threshold temperature at which a reaction becomes nonspontaneous.
Understanding this threshold temperature has practical applications in various fields, including chemical engineering, materials science, and environmental chemistry.
As we delve deeper into this topic, we will uncover the intricate interplay between enthalpy, entropy, and Gibbs free energy, and their profound impact on reaction spontaneity. By gaining a comprehensive understanding of these concepts, we can unravel the mysteries of nonspontaneous reactions and harness their potential for scientific advancements.
Overview of Nonspontaneous Reactions
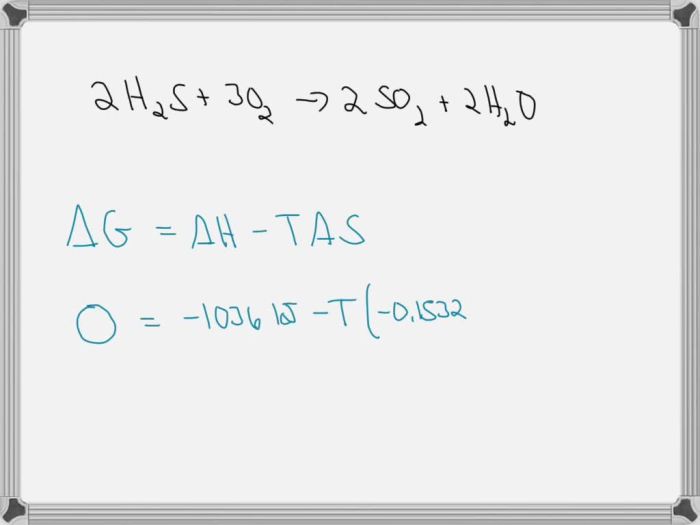
Nonspontaneous reactions are chemical reactions that do not occur naturally without the input of external energy. They require an external force, such as heat or electricity, to drive the reaction forward.
The spontaneity of a reaction is determined by several factors, including enthalpy (heat change), entropy (disorder), and Gibbs free energy (a combination of enthalpy and entropy). A reaction is spontaneous if the Gibbs free energy change is negative, and nonspontaneous if it is positive.
Temperature Dependence of Reaction Spontaneity
Temperature plays a crucial role in determining the spontaneity of a reaction. As temperature increases, the entropy of the system increases, making the reaction more likely to be spontaneous. However, the enthalpy change may also increase with temperature, opposing the effect of entropy.
The overall effect of temperature on spontaneity is determined by the Gibbs free energy change. As temperature increases, the Gibbs free energy change becomes less negative and eventually becomes positive, indicating that the reaction becomes nonspontaneous.
Identifying the Threshold Temperature
The threshold temperature is the temperature at which a reaction becomes nonspontaneous. It can be determined using thermodynamic data, such as enthalpy and entropy changes.
The threshold temperature can be calculated using the equation: “` Threshold temperature = ΔH / (ΔS + R) “` where: – ΔH is the enthalpy change – ΔS is the entropy change – R is the ideal gas constant
Applications of Threshold Temperature Analysis, Above what temperature does the following reaction become nonspontaneous
Understanding the threshold temperature has practical applications in various fields, including:
- Chemical engineering:Designing chemical processes to operate at optimal temperatures for maximum efficiency and yield.
- Materials science:Determining the stability of materials at different temperatures, informing material selection and design.
- Environmental chemistry:Predicting the fate and transport of pollutants in the environment, considering temperature variations.
Questions Often Asked: Above What Temperature Does The Following Reaction Become Nonspontaneous
What is a nonspontaneous reaction?
A nonspontaneous reaction is a chemical reaction that does not occur spontaneously under specified conditions, typically requiring an external energy input to proceed.
How does temperature affect reaction spontaneity?
Temperature affects reaction spontaneity by influencing the Gibbs free energy change. As temperature increases, the Gibbs free energy change becomes less negative, making the reaction less spontaneous.
How can we determine the threshold temperature for a nonspontaneous reaction?
The threshold temperature can be determined by calculating the temperature at which the Gibbs free energy change is zero. This can be done using thermodynamic data, such as enthalpy and entropy changes.